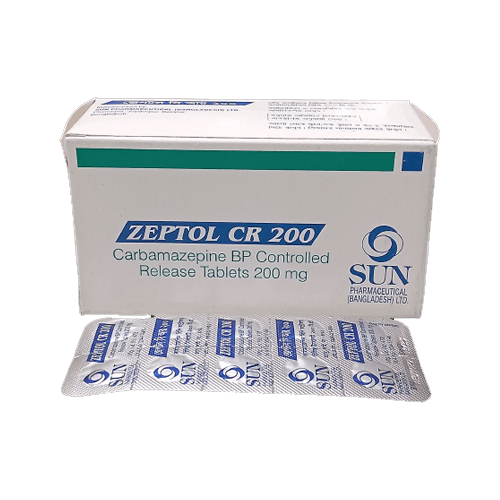



Zeptol CR 200 Tablet - (200mg)
(0
reviews)
Sold by
Ashik Medical
Price
৳64.00
৳70.00
/pc
-9%
Club Point:
20
Refund
Not Applicable
Share
Top Selling Products
-
৳76.50
৳85.00 -
৳27.00
৳30.00 -
৳126.00
৳140.00 -
৳450.00
৳500.00 -
৳135.00
৳150.00 -
৳64.00
৳70.00
Reviews & Ratings
0
out of 5.0
(0
reviews)
There have been no reviews for this product yet.
Introduction
Zeptol CR 200 is an anti-epileptic medicine used to treat epilepsy. It helps prevent certain types of seizures (fits). It is also prescribed for a painful condition of the face, head, and neck known as trigeminal neuralgia and diabetes-related nerve pain (diabetic neuropathy). Zeptol CR 200 is also occasionally used to treat certain serious mood disorders (eg. bipolar disorder) when other medicines have not worked. The dose and how often you need to take it will be decided by your doctor so that you get the right amount to control your symptoms. It may be increased gradually. Many other medicines can interfere with this medicine so tell your doctor about all the medicines you are taking to make sure it is safe. You can take this medicine with or without food but take it regularly at the same time each day to get the maximum benefit. It is important to take this medicine for as long as you are advised, even if you feel well. Missing even a single dose may trigger a seizure and, if you stop taking it abruptly, your condition may get worse. The most common side effects of this medicine include nausea, vomiting, feeling dizzy, tired or drowsy, unsteadiness (balance disorder), constipation, dry mouth, and itching. Some people may develop blurring of vision and slurred speech. Most of the side effects are not serious. However, let your doctor know straight away if you notice a skin rash or if your mood becomes depressed or if you develop any thoughts about harming yourself. Before taking Zeptol CR 200, tell your doctor if you have any heart problems, kidney or liver disease, difficulty in urinating, epilepsy (seizure disorder or fits), or any mental illness like depression. These conditions may affect your treatment. You may be advised some blood tests (eg. CBC) before starting treatment and then periodically thereafter, to monitor your progress.
Uses of Zeptol CR 200
- Epilepsy/Seizures
- Trigeminal neuralgia
Side effects of Zeptol CR 200
Common
- Balance disorder (loss of balance)
- Dizziness
- Drowsiness
- Nausea
- Slurred speech
- Vomiting
- Blurred vision
- Constipation
- Dryness in mouth
- Fatigue
- Itching
How to use Zeptol CR 200
Take this medicine in the dose and duration as advised by your doctor. Swallow it as a whole. Do not chew, crush or break it. Zeptol CR 200 may be taken with or without food, but it is better to take it at a fixed time.
How Zeptol CR 200 works
Zeptol CR 200 is an antiepileptic medication. It controls seizures or fits by decreasing the abnormal and excessive activity of the nerve cells in the brain.
What if you forget to take Zeptol CR 200?
If you miss a dose of Zeptol CR 200, take it as soon as possible. However, if it is almost time for your next dose, skip the missed dose and go back to your regular schedule. Do not double the dose.
Quick Tips
- Take your medication regularly as directed by your doctor as missing doses can trigger seizures.
- Do not change the brand of your medicine and make sure that you have sufficient amount of medicine present with you.
- Some healthy tips to prevent seizures:
- It may cause dizziness and sleepiness. Do not drive or do anything that requires mental focus until you know how it affects you.
- Your doctor may get regular tests done to monitor the level of blood cells in your blood while taking this medication.
- Talk to your doctor if you notice sudden mood changes or develop suicidal thoughts.
- Inform your doctor if you notice a rash or other skin changes such as reddish spot or circular patches while taking this medicine.
- Do not stop taking the medication suddenly without talking to your doctor as it may increase the seizure frequency.
Brief Description
Indication
Epilepsy, Schizophrenia, Bipolar disorder, Trigeminal neuralgia
Administration
Should be taken with food. Avoid grapefruit juice.
Adult Dose
Oral Epilepsy Adults - Initial: Either 200 mg b.i.d. for tablets and XR tablets, or 1 teaspoon q.i.d. for suspension (400 mg/day). Increase at weekly intervals by adding up to 200 mg/day using a b.i.d or a t.i.d. or q.i.d. regimen of the either formulations until the optimal response is obtained. Doses up to 1600 mg daily have been used in adults in rare instances. Maintenance: usually 800-1200 mg daily. Trigeminal neuralgia Adult: Initially, 100-200 mg bid, increased gradually as needed. Maintenance: 400-800 mg daily in divided doses. Max: 1.2 g daily. Prophylaxis of bipolar disorder Adult: Initially, 400 mg daily in divided doses, increased gradually as necessary. Maintenance: 400-600 mg daily in divided doses. Max: 1.6 g daily.
Child Dose
Epilepsy <6 Years Initial (oral suspension): 10-20 mg/kg/day PO q6hr Initial (tablet): 10-20 mg/kg/day PO q8-12hr Maintenance: For tablets or suspension may divide frequency into 3-4 times daily not to exceed 35 mg/kg/day 6-12 Years Initial (oral suspension): 50 mg PO q6hr Initial (tablet, immediate- or extended-release): 100 mg PO q12hr; may increase qWeek by 100 mg/day Maintenance: 400-800 mg/day PO q6-8hr (immediate-release); q12hr (extended-release) Not to exceed 1000 mg/day >12 Years Initial (oral suspension): 10 mL (200 mg) PO q6hr Initial (tablet, immediate- or extended-release): 200 mg PO q12hr May increase by up to 200 mg/day qWeek; q12hr (extended-release tablet); q6-8hr (other formulations) 12-15 years: Dose not to exceed 1000 mg/day >15 years: Dose not to exceed 1200 mg/day
Contraindication
Hypersensitivity; bone marrow depression; porphyria, pregnancy.
Mode of Action
Carbamazepine reduces polysynaptic responses and blocks post-tetanic potentiation. It is effective in partial and generalised convulsions as well as in mixed types but not in petit mal seizures. It reduces or abolishes pain in trigeminal and glossopharyngeal neuralgia.
Precaution
Lactation; CV disease, hepatic or renal disorders, history of blood disorders or haematological reactions to other drugs; glaucoma; skin disorders; elderly, patients on MAO inhibitors; abrupt withdrawal of treatment. Lactation: Enters breast milk; not recommended (AAP states compatible with nursing; however, adverse reactions in breastfeeding infant are possible; take into account the importance of the drug to the mother before deciding to discontinue breastfeeding or the drug)
Side Effect
>10% Ataxia (15%),Dizziness (44%),Drowsiness (32%),Nausea (29%),Vomiting (18%) 1-10% Dry mouth (8%) Rare MI,Stevens-Johnson syndrome Hepatic failure,Punctate cortical lens opacities,Syndrome of inappropriate antidiuretic hormone secretion (SIADH) Frequency Not Defined Hemopoietic system: Aplastic anemia, agranulocytosis, pancytopenia, bone marrow depression, thrombocytopenia, leukopenia, leukocytosis, eosinophilia, anemia, acute intermittent porphyria, variegate porphyria, porphyria cutanea tarda Skin: Toxic epidermal necrolysis (TEN) and Stevens-Johnson syndrome (SJS) (see Black Box Warnings), pruritic and erythematous rashes, urticaria, photosensitivity reactions, alterations in skin pigmentation, exfoliative dermatitis, erythema multiforme and nodosum, purpura, aggravation of disseminated lupus erythematosus, alopecia, diaphoresis, and onychomadesis Cardiovascular system: Congestive heart failure, edema, aggravation of hypertension, hypotension, syncope and collapse, aggravation of coronary artery disease, arrhythmias and AV block, thrombophlebitis, thromboembolism, and adenopathy or lymphadenopathy Liver: Abnormalities in liver function tests, cholestatic and hepatocellular jaundice, hepatitis; very rare cases of hepatic failure Pancreatic: Pancreatitis Respiratory System: Pulmonary hypersensitivity characterized by fever, dyspnea, pneumonitis, or pneumonia Genitourinary System: Urinary frequency, acute urinary retention, oliguria with elevated blood pressure, azotemia, renal failure, and impotence (rare reports of impaired male fertility and/or abnormal spermatogenesis) Laboratory: Albuminuria, glycosuria, elevated BUN, decreased plasma calcium, and microscopic deposits in the urine Nervous system: Dizziness, drowsiness, disturbances of coordination, confusion, headache, fatigue, blurred vision, visual hallucinations, transient diplopia, oculomotor disturbances, nystagmus, speech disturbances, abnormal involuntary movements, peripheral neuritis and paresthesias, depression with agitation, talkativeness, tinnitus, hyperacusis, neuroleptic malignant syndrome; isolated cases of neuroleptic malignant syndrome Digestive system: Nausea, vomiting, gastric distress and abdominal pain, diarrhea, constipation, anorexia, and dryness of the mouth and pharynx, including glossitis, and stomatitis Eyes: Scattered punctate cortical lens opacities, increased intraocular pressure as well as conjunctivitis Musculoskeletal system: Aching joints and muscles, and leg cramps Metabolism: Fever and chills; SIADH; cases of frank water intoxication, with decreased serum sodium (hyponatremia) and confusion; decreased levels of plasma calcium leading to osteoporosis Potentially Fatal: Agranulocytosis, aplastic anaemia, hepatic failure, severe exfoliative dermatitis and Stevens-Johnson syndrome.
Pregnancy Category Note
Pregnancy category: D Lactation: Enters breast milk; not recommended (AAP states compatible with nursing; however, adverse reactions in breastfeeding infant are possible; take into account the importance of the drug to the mother before deciding to discontinue breastfeeding or the drug)
Interaction
Increased plasma levels w/ CYP3A4 inhibitors (e.g. cimetidine). Decreased plasma levels w/ CYP3A4 inducers (e.g. cisplatin). Increased risk of neurotoxic side effects w/ lithium. May decrease the effect of hormonal contraceptives. Increased plasma levels of active metabolite carbamazepine-10, 11-epoxide w/ loxapine, quetiapine, primidone, progabide, valproic acid and valpromide. May increase cyclophosphamide levels. May reduce exposure of aripiprazole. May reduce plasma levels of tacrolimus, temsirolimus and lapatinib. May increase risk of isoniazid-induced hepatotoxicity. Risk of symptomatic hyponatraemia w/ diuretics (e.g. hydrochlorothiazide, furosemide). Potentially Fatal: May decrease serum concentrations of nefazodone and its active metabolites. Toxic reactions may develop when taken concurrently w/ MAOIs.
Frequently Bought Products
Product Queries (0)
Login Or Registerto submit your questions to seller
Other Questions
No none asked to seller yet
Top Selling Products
-
৳76.50
৳85.00 -
৳27.00
৳30.00 -
৳126.00
৳140.00 -
৳450.00
৳500.00 -
৳135.00
৳150.00 -
৳64.00
৳70.00