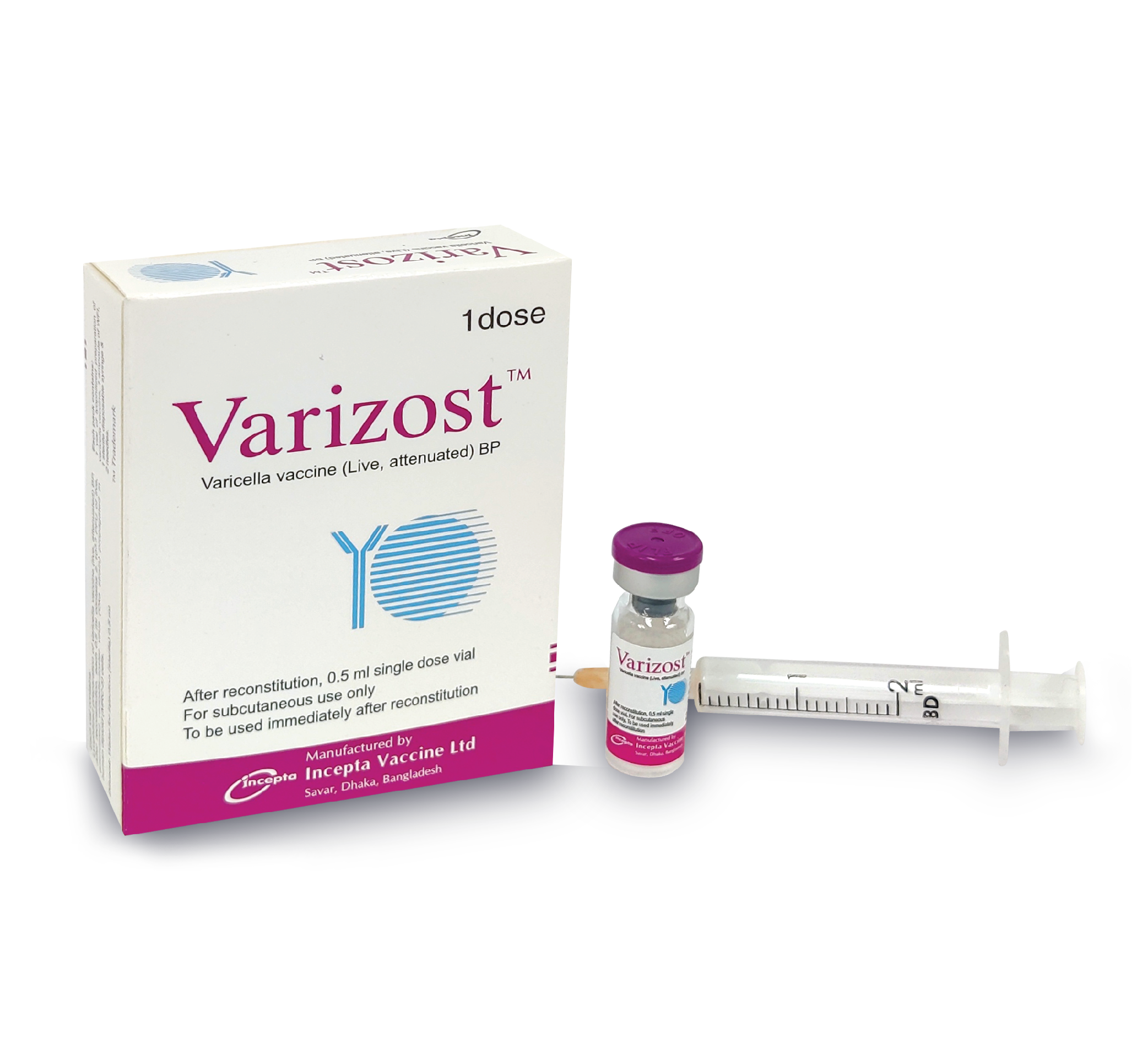



Varizost Injection - (0.5 ml/vial)
(0
reviews)
Sold by
Ashik Medical
Price
৳1,260.00
৳1,400.00
/pc
-10%
Club Point:
200
Refund
Not Applicable
Share
Top Selling Products
-
৳450.00
৳500.00 -
৳64.00
৳70.00 -
৳36.11
৳40.12 -
৳94.50
৳105.00 -
৳12.42
৳13.80
Reviews & Ratings
0
out of 5.0
(0
reviews)
There have been no reviews for this product yet.
Indication
Chickenpox
Adult Dose
Subcutaneous Active immunisation against varicella infection Adult: >12yr: 2 doses of 0.5 ml each. 2nd dose to be given at least 4 weeks later after the 1st dose. Postexposure vaccination Adult: >12 yr: Given within 3-5 days post exposure, a single dose of 0.5 ml. 2nd dose to be given 4 wk after the 1st dose.
Child Dose
Subcutaneous Active immunisation against varicella infection Child: >12yr: 2 doses of 0.5 ml each. 2nd dose to be given at least 4 weeks later after the 1st dose. Child: 1-12 yr: 0.5 ml, as a single dose. If necessary, a 2nd dose is to be given at least 3 mth later. Postexposure vaccination Child: >12 yr: Given within 3-5 days post exposure, a single dose of 0.5 ml. 2nd dose to be given 4 wk after the 1st dose. Child: 1-12 yr: Given within 3-5 days post exposure, a single dose of 0.5 ml. If necessary, a 2nd dose is to be given at least 3 mth later. Varicella Immunization Indicated for routine immunization (ACIP guidelines) Minimum age: 12 months Routine vaccination 2 dose series: 0.5 mL SC 1st dose: 12-15 months 2nd dose: 4-6 years; may be administered before age 4 yr, provided at least 3 months have elapsed since the first dose
Contraindication
Hypersensitivity to neomycin, gelatin or other components of the vaccine. Malignant neoplasm of the bone marrow or lymphatic systems. Patients who are currently on immunosuppressants. Primary or acquired immunodeficiency states. Active untreated tuberculosis, active febrile infection (>38.5°C>. Pregnancy and avoid pregnancy for 1-3 mth following vaccination.
Mode of Action
Live, attenuated varicella virus stimulates active immunity to disease caused by varicella-zoster virus. Conveys active immunity via stimulation of production of endogenously produced antibodies .
Precaution
Vaccination should be deferred for at least 5-11 mth following blood or plasma transfusion, or admin of immune globulin. Delay admin of vaccine for at least 1 mth after stopping high dose immunosuppressive therapy. Avoid close contact with high risk individuals for up to 6 wk. Lactation: Not known if excreted in breast milk; use caution
Side Effect
>10% Injection site swelling/rash/pruritus/erythema (20%) Fever >102°F [39°C] (15%) Potentially Fatal: Anaphylaxis, Stevens-Johnson syndrome.
Interaction
Avoid use of salicylates for 6 wk after vaccination to reduce risk of Reye's syndrome. Decreased efficacy of vaccine with antiviral agents active against herpes virus (e.g. aciclovir, valaciclovir). Decreased immnuologic response to live vaccines if patients on immunosuppressive agents e.g. high dose corticosteroids, alkylating agents, antimetabolites, radiation therapy.
Frequently Bought Products
Product Queries (0)
Login Or Registerto submit your questions to seller
Other Questions
No none asked to seller yet
Top Selling Products
-
৳450.00
৳500.00 -
৳64.00
৳70.00 -
৳36.11
৳40.12 -
৳94.50
৳105.00 -
৳12.42
৳13.80