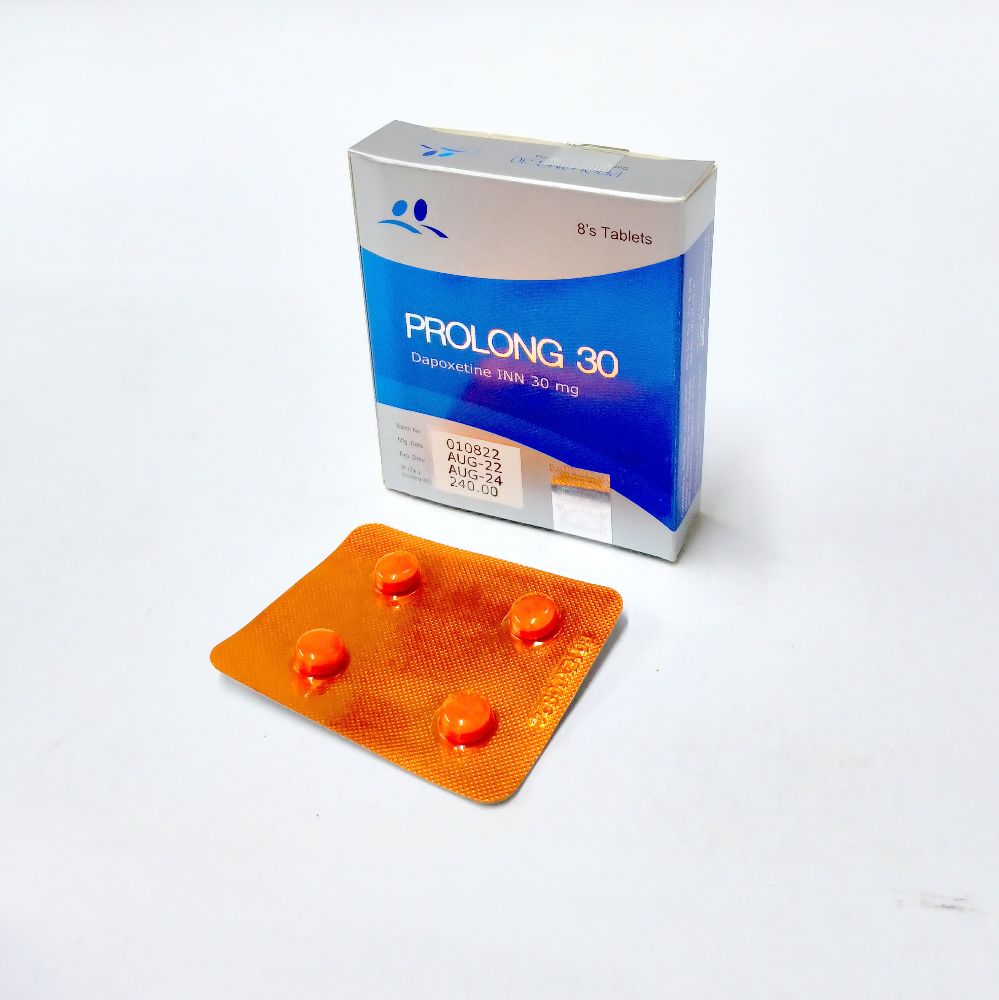

Prolong 30 - 1 strip
(0
reviews)
Sold by
Ashik Medical
Price
৳108.00
৳120.00
/pc
-10%
Club Point:
50
Refund
Not Applicable
Share
Top Selling Products
-
৳450.00
৳500.00 -
৳64.00
৳70.00 -
৳36.11
৳40.12 -
৳94.50
৳105.00 -
৳12.42
৳13.80
Reviews & Ratings
0
out of 5.0
(0
reviews)
There have been no reviews for this product yet.
Medicine Overview of Prolong 30mg Tablet
Introduction
Prolong 30 is a medicine used to treat premature ejaculation in adult men. This medicine increases the time it takes to ejaculate and improve control over ejaculation. This helps to relieve the anxiety or frustration about fast ejaculation. Prolong 30 belongs to a group of medicines known as selective serotonin reuptake inhibitors (SSRI). It may be taken with or without food. This medicine should only be taken if you have been diagnosed with premature ejaculation by a doctor. It is usually advised to be taken 1 to 3 hours before anticipated sexual activity. Do not take it more than once in a day. Follow your doctor's instructions while taking this medicine. Some common side effects of this medicine include headache, dizziness, drowsiness, vomiting, nausea, indigestion, fatigue, increased sweating, and restlessness. Some people can develop erectile dysfunction with this medicine. Let your doctor know if any of the side effects persist or worry you. Do not drive or do anything that requires focus (eg. working with heavy machinery) until you know how this medicine affects you, as some people can develop dizziness and sleepiness as side effects. Do not take Prolong 30 if you have heart problems (like heart failure or problems with the heart rhythm), or have ever had depression or mania, or are currently taking medicines for depression known as MAO inhibitors. Before taking it, you should tell your doctor if you have epilepsy (seizure disorder or fits), liver or kidney disease, glaucoma, or have had dizziness/fainting (syncope) due to low blood pressure in the past. These may affect your treatment.
Uses of Prolong 30
- Premature ejaculation
Side effects of Prolong 30
Common
- Drowsiness
- Nausea
- Tremor
- Vomiting
- Dizziness
- Erectile dysfunction
- Fatigue
- Headache
- Increased sweating
- Indigestion
- Restlessness
How to use Prolong 30
Take this medicine in the dose and duration as advised by your doctor. Swallow it as a whole. Do not chew, crush or break it. Prolong 30 may be taken with or without food, but it is better to take it at a fixed time.
How Prolong 30 works
Prolong 30 is a selective serotonin reuptake inhibitor (SSRI) which increases the level of serotonin in the nervous system to increase the time taken to ejaculate and improve control over ejaculation.
What if you forget to take Prolong 30?
If you miss a dose of Prolong 30, take it as soon as possible. However, if it is almost time for your next dose, skip the missed dose and go back to your regular schedule. Do not double the dose.
Quick Tips
- Prolong 30 should only be taken 1 to 3 hours before sexual activity is anticipated.
- Do not take this medicine more than once 24 hours or every day.
- Do not drive or do anything requiring concentration until you know how Prolong 30 affects you.
- It should be used with caution in patients with history of seizures, depression and other psychiatric disorders.
- Prolong 30 may cause a sudden drop in your blood pressure. Rise slowly if you have been sitting or lying down.
- Talk to your doctor before taking this medicine if you are suffering from erectile dysfunction.
Brief Description
Indication
Premature ejaculation
Administration
May be taken with or without food. Swallow tab whole & take w/ a full glass of water.
Adult Dose
Oral Premature Ejaculation Adult: 18-64 yr Initially, 30 mg approx 1-3 hr before sexual activity, subsequent doses adjusted according to response. Max: 60 mg. Dose should not be taken more than once in 24 hr. Review treatment after 4 wk (6 doses) and at least 6 mthly thereafter. Hepatic Impairment: Moderate to severe: Contraindicated.
Renal Dose
Patients with Renal Impairment: No dose adjustment is required but caution is advised in patients with mild or moderate renal impairment. Dapoxetine is not recommended for use in the patients with severe renal impairment.
Contraindication
Known hypersensitivity to dapoxetine HCl or to any of the excipients. Patients with significant pathological cardiac conditions [eg, heart failure (NYHA class II-IV), conduction abnormalities (2nd- or 3rd-degree AV block or sick sinus syndrome) not treated with a permanent pacemaker, significant ischemic heart disease or significant valvular disease]. Concomitant treatment with monoamine oxidase inhibitors (MAOIs), or within 14 days of discontinuing treatment with a MAOI. Similarly, a MAOI should not be administered within 7 days after Dapoxetine has been discontinued. Concomitant treatment with thioridazine, or within 14 days of discontinuing treatment with thioridazine. Similarly, thioridazine should not be administered within 7 days after Dapoxetine has been discontinued. Concomitant treatment with serotonin re-uptake inhibitors [selective serotonin re-uptake inhibitors (SSRIs), serotonin-norepinephrine re-uptake inhibitors (SNRIs), tricyclic antidepressants (TCAs)] or other medicinal/herbal products with serotonergic effects or within 14 days of discontinuing treatment with these medicinal/herbal products. Similarly these medicinal/herbal products should not be administered within 7 days after Dapoxetine has been discontinued.
Mode of Action
Dapoxetine selectively inhibits the reuptake of serotonin. It has limited direct action at other neurotransmitter sites including muscarinic receptors.
Precaution
General: Dapoxetine is only indicated in men with PE. Safety has not been established and there are no data on the ejaculation-delaying effects in men without PE. Use with Recreational Drugs: Patients should be advised not to use Dapoxetine in combination with recreational drugs. Recreational drugs with serotonergic activity eg, ketamine, methylenedioxymethamphetamine (MDMA) and lysergic acid diethylamide (LSD) may lead to potentially serious reactions if combined with Dapoxetine. These reactions include, but are not limited to, arrhythmia, hyperthermia and serotonin syndrome. Use of Dapoxetine with recreational drugs with sedative properties eg, narcotics and benzodiazepines may further increase somnolence and dizziness. Ethanol: Combining alcohol with Dapoxetine may increase alcohol-related neurocognitive effects and may also enhance neurocardiogenic adverse events eg, syncope, thereby increasing the risk of accidental injury; therefore, patients should be advised to avoid alcohol while taking Dapoxetine. Possibly prodromal symptoms eg, nausea, dizziness/lightheadedness and diaphoresis were reported more frequently among patients treated with Dapoxetine compared to placebo. Orthostatic Hypotension: An orthostatic test should be performed before initiating therapy. In case of a history of documented or suspected orthostatic reaction, treatment with Dapoxetine should be avoided. Dapoxetine is not indicated for psychiatric disorders and should not be used in men with these disorders eg, schizophrenia, or in those suffering with co-morbid depression, as worsening of symptoms associated with depression cannot be excluded. Withdrawal Effects: Abrupt discontinuation of chronically administered SSRIs used to treat chronic depressive disorders has been reported to result in the following symptoms: Dysphoric mood, irritability, agitation, dizziness, sensory disturbances (eg, paresthesias, electric shock sensations), anxiety, confusion, headache, lethargy, emotional lability, insomnia and hypomania.
Side Effect
Syncope characterized as loss of consciousness has been reported.The majority of cases occurred during the first 3 hrs after dosing, after the 1st dose or associated with study-related procedures in the clinic setting (eg, blood draw and orthostatic maneuvers and blood pressure measurements). Prodromal symptoms often preceded the syncope. Orthostatic hypotension has been reported in clinical trials. The most common adverse drug reactions (5%) reported during clinical trials were headache, dizziness, nausea, diarrhea, insomnia and fatigue. The most common events leading to discontinuation were nausea and dizziness.
Interaction
Potential serious reactions w/ MAOIs. Inhibits metabolism of thioridazine. Risk of serotonergic-associated effects w/ serotonergic medicinal/herbal products (including L-tryptophan, triptans, tramadol, linezolid, SSRIs, SNRIs, lithium & St. John's wort prep). CNS-active medicinal products. Possible reduction of clearance w/ CYP2D6, CYP3A4 & flavin monooxygenase 1 inhibitors. Increased exposure w/ potent (eg ketoconazole, itraconazole, ritonavir, saquinavir, telithromycin, nefazodone, nelfinavir & atazanavir) & moderate (eg erythromycin, clarithromycin, fluconazole, amprenavir, fosamprenavir, aprepitant, verapamil, diltiazem) CYP3A4 inhibitors; potent CYP2D6 inhibitors. Possible reduced orthostatic tolerance w/ PDE-5 inhibitors & ?-adrenergic receptor antagonists. Increases plasma conc of desipramine & other drugs metabolized by CYP2D6. Decreases AUCinf of midazolam. Warfarin (chronic therapy). Alcohol.
Frequently Bought Products
Product Queries (0)
Login Or Registerto submit your questions to seller
Other Questions
No none asked to seller yet
Top Selling Products
-
৳450.00
৳500.00 -
৳64.00
৳70.00 -
৳36.11
৳40.12 -
৳94.50
৳105.00 -
৳12.42
৳13.80